Marchetti Lab
ecophysiology, biogeochemistry and genomics of marine phytoplankton
Topics:
1. Iron limitation of phytoplankton
2. Diatoms are young, beautiful and important
3. Using molecular techniques to study microbes in the sea
4. Diatom-bacteria interactions
5. Fragilariopsis kerguelensis: an important polar diatom in the Southern Ocean
Iron limitation of phytoplankton
Phytoplankton come in all shapes and sizes
Phytoplankton are free-drifting photosynthetic cells that are present throughout the sunlit waters of the ocean. They come in all shapes and sizes ranging from the micrometer (10-6m) to millimeter (10-3 m) scales. Similar to plants, they convert light energy from the sun into chemical energy to create their food but differ from plants in that they are single-celled and constitute both prokaryotic and eukaryotic organisms with a range of distinct evolutions.
At any point in time, phytoplankton account for less than 2% of Earth’s total living biomass, however due to their fast turnover rates, over the course of a year, phytoplankton are responsible for approximately 50% of the annual total carbon fixed into organic matter on Earth. In addition to carbon, they require many other nutrients to grow. Elements that are typically found in micromolar concentrations in seawater are called macronutrients. Phytoplankton biomass in the ocean is mainly controlled in two ways. First, phytoplankton are prey for planktonic animals called zooplankton. Second, phytoplankton growth rates could be controlled by physical or chemical properties of the ocean including light, temperature and nutrient contentrations. A very nice overview paper written by Paul Falkoski from Rutgers University that discusses the importance of phytoplankton can be found here.
Nitrogen (N), phosphorus (P) and silicon (Si) are macronutrients commonly limiting to phytoplankton growth in the ocean. Diatoms, a major group of phytoplankton, are rather unique in that they require silicon to build their glass shells known as frustules. Because of the large number of phytoplankton biomass made up of diatoms, they are the most important group influencing the cycling of silicon in the ocean. Elements that are found in concentrations much lower than micromolar quantities are called micronutrients. The most common growth limiting micronutrient to phytoplankton in the ocean is iron.
Iron is essential for cell growth, metabolism and function. Phytoplankton have an obligate need for iron as components of numerous proteins and enzymes. In many areas of the ocean, the availability of iron is low enough that it limits phytoplankton growth. In fact, iron is the primary nutrient limiting phytoplankton growth in 30-40% of the world’s oceans. These areas are usually far from landmasses that are the primary source of new iron to the ocean. There are three main iron-limited regions on Earth. They are the North Pacific, the Equatorial Pacific and the Southern Oceans. Because these iron-limited regions of the ocean have high concentrations of macronutrients (e.g. N, Si and P) yet low concentrations of chlorophyll (a proxy for phytoplankton biomass), these areas are referred to as High-Nutrient, Low-Chlorophyll (HNLC) regions.
Since the first evidence showing the extent to which phytoplankton growth may be limited by iron in the ocean, many researchers have examined the critical functions iron has within the cell and the abilities of phytoplankton from low-iron regions to cope with it’s limited availability. For example, oceanic diatoms from HNLC regions are extremely efficient at lowering their iron requirements and have adopted numerous iron uptake strategies. This means that oceanic diatoms have adapted to living in low iron environments (see below).
Given that iron is limiting in the ocean but is in abundant supply on land, it has been proposed that iron fertilization could be a way of combating climate change. You might be asking what is the connection between iron fertilization and climate change? Well, the concept goes like this:
1. Phytoplankton in certain regions of the ocean are iron-limited. If we fertilize these areas with iron (in the same way you add fertilizer to your garden to increase plant growth) we could increase the growth of phytoplankton.
2. When phytoplankton grow, they take up carbon dioxide in the water and use it to build cell components, thus turning it into organic matter. When these phytoplankton die, they sink out of the sunlit parts of the ocean. Although much of this organic matter is remineralized back into carbon dioxide by bacteria, some of these cells make it to deep ocean depths and sediments where they can trap the carbon left inside them for hundreds to thousands of years.
3. If carbon from the upper parts of the ocean is transported to depth, more carbon dioxide from the atmosphere is sucked into the upper ocean. Increased carbon dioxide in the atmosphere is thought to be the leading cause of climate change. Because iron fertilization could result in reduced atmospheric carbon dioxide concentrations, it is being considered as a geo-engineering strategy to slow down climate change.
For more information about iron fertilization there are some links to other websites you can visit on the links webpage.
Diatoms are young, beautiful and important
In comparison to the other dominant groups of phytoplankton in the ocean, diatoms are young. Fossils of diatoms first emerge in sediments on the ocean floor roughly 200 million years ago (Ma). This is long after other phytoplankton made their first appearances in the fossil record. Diatoms are divided into two major groups that differ in their morphology, physiology and modes of reproduction. Centric diatoms are radially symmetric and are shaped like wheels, whereas pennate diatoms are bilaterally symmetric and are shaped like needles. Early forms of diatoms were of the centric variety and were confined to shallow marine habitats. A major divergence in diatoms occurred about 75 Ma to generate pennate diatoms. Centric diatoms first appear in offshore, pelagic ocean waters about 65 Ma despite the chronically low levels of nutrients, in particular iron. Pennate diatoms do not appear to have moved to offshore waters until roughly 20 Ma.
In their relatively short existence, diatoms have evolved rapidly. They are ubiquitous to all aquatic environments ranging from backyard ponds to the nutrient-deprived open ocean. Their importance in the cycling of elements and carbon cannot be understated. They are estimated to account for roughly half of total marine primary productivity which equates to about a quarter of global primary productivity. In addition, because their intricately shaped frustules are comprised of silicon, it is believed that every atom of silicon in the ocean has been incorporated into a diatom frustule 39 times before being buried in the ocean floor. An excellent video displaying the amazing shapes and sizes of diatoms can be viewed here. Diatoms are also at the base of marine food webs and are particularly important in highly productive coastal waters and polar regions where they form large blooms that can be observed by satellites far above the Earth’s surface.
There is a large diversity of diatoms present in open ocean waters, although typically rare members of the overall phytoplankton assemblage, . As diatoms are considered the largest of all phytoplankton groups, it is thought they might be at a disadvantageous in areas where nutrients are scarce because the nutrient demand of a cell usually scales with it’s size. Therefore the bigger the cell, the more nutrients it requires. However despite their relatively large size, oceanic diatoms found in low iron regions of the ocean have adapted well to these conditions. There are a number of strategies they have invoked to deal with reduced iron availability. Firstly, oceanic diatoms have reduced their iron requirements by streamlining their proteomes. By minimizing proteins that require iron, this enables oceanic diatoms to grow faster under low iron conditions that even the smallest of coastal diatoms could not tolerate.
Secondly, diatoms have the ability to perform luxury uptake. Luxury uptake is the accumulation of intracellular iron concentrations in excess of what the cell needs to grow, essentially resulting in an iron reserve or storage. In pennate diatoms, the ability to perform luxury uptake is mediated through the iron storage protein ferritin. As a result of their large storage capacities, the iron contents of oceanic pennate diatoms from iron-limited regions grown in nutrient-replete conditions may be up to 60 times that of what they are under iron-limiting conditions. Having the ability to store iron comes in handy when there are sporadic inputs of iron by way of either enhanced mixing of iron-rich deep waters or dust deposition from the atmosphere.
Lastly, certain oceanic diatoms contain proteorhodopsins. In the marine environment, proteorhodopsins are generally considered prokaryotic proteins that act as light sensors for phototaxis or as light-driven proton pumps that generate a proton motive force to drive synthesis of the energy molecule ATP, cell motility, and/or import of small molecules. Recently, proteorhodospin has been proposed to fuel light-driven ATP synthesis in eukaryotes based on the identification of rhodopsin-type genes in several heterotrophic and mixotrophic dinoflagellates. The presence of rhodopsins in diatoms is surprising as they contain chloroplasts that provide all the necessary machinery for oxygenic photosynthesis, which is a much more effective way of producing ATP and other molecules required for carbon fixation. However due to the high iron requirements in photosynthesis and lack of trace metals required in rhodopsins, they may be important in the synthesis of ATP in low-iron environments.
Using molecular techniques to study microbes in the sea
 Environmental transcriptomics (metatranscriptomics) is the sequencing of RNA by high-throughput sequencing technologies that enables identification of expressed genes of microorganisms within the natural environment. Because species have distinct genetic codes, sequences can be categorized into specific taxonomic groups based on their similarity to sequences from reference organisms. Laboratory studies with model microorganisms have shown that expression of many genes is altered by changes in growth conditions.
Environmental transcriptomics (metatranscriptomics) is the sequencing of RNA by high-throughput sequencing technologies that enables identification of expressed genes of microorganisms within the natural environment. Because species have distinct genetic codes, sequences can be categorized into specific taxonomic groups based on their similarity to sequences from reference organisms. Laboratory studies with model microorganisms have shown that expression of many genes is altered by changes in growth conditions.
The expression patterns of numerous genes that belong to specific metabolic pathways provide a way to figure out how different environmental conditions influence global cellular processes. For example, when nitrogen-limited cells are resupplied with nitrate, transcripts of genes encoding proteins for nitrate transporters, nitrate and nitrite reductases, glutamate and glutamine synthases and numerous other steps involved in nitrogen assimilation are up-regulated. Thus, metatranscriptomics can lend valuable insights into the diversity and ecology of metabolically active organisms in nature and may also be a useful tool in evaluating how these organisms are affected by environmental changes.
Until recently, the high costs associated with sequencing and the low number of sequenced reference genomes restricted the application of performing metatranscriptomic surveys of marine microeukaryotes. Ultimately, the number and breadth of organismal reference sequences dictates the success in being able to assign a taxonomic and gene function annotation to sequences obtained from the environment. Meta-omic approaches have benefited from recent advancements in sequencing technologies that produce large sequence libraries of relatively short sequence read lengths at affordable costs.
Sequencing efforts of whole genomes and Expressed Sequence Tags (ESTs) from a variety of marine microeukaryotes have greatly increased our ability to annotate sequences collected from mixed plankton assemblages. These databases now include representatives from each of the major phytoplankton groups: heterokontophyta (diatoms and non-diatom members), haptophytes, cryptophytes, chlorophytes and dinoflagellates. Additionally, the Marine Microbiology Initiative funded by the Gordon and Betty Moore Foundation has recently initiated sequencing of 750 EST libraries specifically targeting marine microeukaryotes that will greatly expand the number of existing reference sequence databases. I discuss this program in more detail here.
Diatom-bacteria interactions
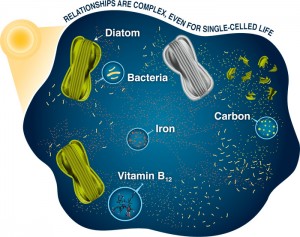
Biological oceanographers are just beginning to explore the unique association between diatoms and bacteria, yet it is already clear that a complex relationship exists that consists of signaling mechanisms and nutrient exchanges. On one hand, diatoms are a major group of phytoplankton that perform much of the photosynthesis in the sea. They are the base of many food chains of which life in the ocean is dependent upon, including some types of bacteria (Ambrust, 2009). Due to their heavily silicified shells, diatoms tend to sink out of the surface rapidly and export organic matter to the deep ocean. Conversely, bacteria utilize the organic carbon produced by these photosynthesizers and perform a process called remineralization in which organic matter is converted back to its inorganic components and reintroduced into the water column. Thus, both diatoms and bacteria play a major role in the biological pump, carbon cycle, and biogeochemical cycles of important nutrients. The diatom-bacteria relationship is especially interesting since they have evolved together over time. The genomes of phytoplankton even harbor many bacterial genes, confirming this intimate relationship (Bowler et al., 2008).
There are multiple studies documenting the phenomenon of diatoms living in association with marine bacteria. Reasons for the association include parasitism, with bacteria even emitting algicidal compounds (Amin et al., 2012). In these scenarios marine bacteria thrive on organic matter released from destroyed diatom cells. However mutualisms between the two organisms may exist as well, in which both parties exchange and acquire nutrients, vitamins, trace metals, or dissolved carbon.
There appears to be a mutualistic relationship between diatoms and bacteria concerning the exchange of vitamins. Since diatoms cannot produce vitamin B12 themselves, they must obtain it from the environment. Diatoms must depend on the B12 produced and emitted by bacteria, and the very presence of diatoms can even stimulate its synthesis (Croft et al. 2005). Perhaps since diatoms are dependent upon bacteria to provide them with B12, and there are other vitamins produced by diatoms (such as vitamin B7) or organics that bacteria may require, mutualistic associations have evolved due to the advantage of being in close proximity.
 Several studies have demonstrated that bacteria and diatoms have a number of mechanisms for physically attaching to one other in the vast ocean. For instance, marine bacteria can adjust their swimming speed when phytoplankton are present in an attempt to increase the number of encounters (Barbara et al. 2003). Additionally, diatoms and bacteria both secrete organic compounds that may function in facilitating attachment to one another (Gardes et al 2011, Rinto-Kanta et al 2012).
Several studies have demonstrated that bacteria and diatoms have a number of mechanisms for physically attaching to one other in the vast ocean. For instance, marine bacteria can adjust their swimming speed when phytoplankton are present in an attempt to increase the number of encounters (Barbara et al. 2003). Additionally, diatoms and bacteria both secrete organic compounds that may function in facilitating attachment to one another (Gardes et al 2011, Rinto-Kanta et al 2012).
An especially exciting area of research is the inter-domain communication between diatoms and bacteria. Diatoms are able to produce pheromones that function in sexual reproduction, while bacteria are known to participate in quorum sensing, in which released compounds called autoinducers regulate population density. Current efforts are to investigate whether these signaling molecules can cross-function as a communication tool between the two different groups. Illustrating this point is a study that found the marine bacterium Vibiro paahaemolyticus, a pathogen of marine squid, responds to the squid’s secreted nitric oxide signaling molecule by in turn triggering pathogenic colonization (Wang et al., 2010). Thus there is evidence for communication between bacteria and animals, and there likely exists signaling molecules exuded by diatoms that may be perceived by bacteria and vice versa.
In the Marchetti Lab, we explore diatom-bacteria interactions by investigating how environmental factors, such as the presence or absence of marine bacteria, may affect the production of vitamin B7, or biotin, in diatoms. Many marine bacteria are unable to produce biotin themselves and must scavenge it from seawater, while diatoms are unable to produce vitamin B12. If diatoms are capable of perceiving bacteria through chemical signals, perhaps biotin is being produced in excess quantities in an effort to obtain vitamin B12 from their bacterial counterparts. To learn more about this project and its implications, click here.
References:
Armbrust, V.E (2009) The life of diatoms in the world’s oceans Nature 459:185-192.
Amin S.A., Parker M.S., Armbrust, E.V (2012) Interactions between diatoms and bacteria MMBR 76: 667-684.
Bowler C., et al. (2008) The Phaeodactylum genome reveals the evolutionary history of diatom genomes Nature 456:239-244.
Croft M.T., et al. (2005) Algae acquire vitamin B12 through a symbiotic relationship with bacteria Nature 438:90-93.
Barbara G.M., et al. (2003) Bacterial tracking of motile algae FEMS Microbiol. Ecol. 44:79-87.
Gardes A., et al. (2011). Diatom-associated bacteria are required for aggregation of Thalassiosira weissflogii ISME J 5:436-445.
Rinta-Kanto J.M., et al. (2012) Bacterial community transcription patterns during a marine phytoplankton bloom. Environ. Microbiol. 14:228-239.
Wang Y., et al. (2010) H-NOX-mediated nitric oxide sensing modulates symbiotic colonization by Vibrio fisheri PNAS 107:8375-8380.
Image by Amy Caracappa-Qubeck, Woods Hole Oceanographic Institution
Fragilariopsis kerguelensis: an important polar diatom in the Southern Ocean
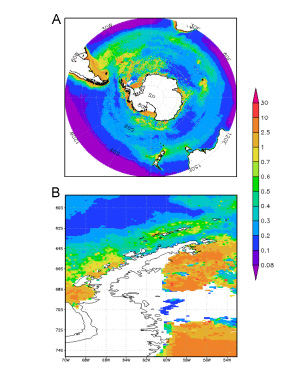
Austral summer climatologically-averaged (Nov-Feb) chlorophyll a distributions in the A) Southern Ocean surface waters and B) Western Antarctic Peninsula surrounding surface waters obtained by SeaWiFS. Color bar in mg Chl m-3. Data provided by NASA Giovanni Portal.
The Southern Ocean is an important ecosystem in the world’s oceans in terms of primary productivity, driving ocean circulation, and biogeochemical cycles. It is a broad, oceanographic region that surrounds Antarctica and encompasses the southern portions of the Pacific, Atlantic and Indian Oceans. The Southern Ocean is characterized by complex oceanographic zones and fronts that extend from the waters off the coast of Antarctica to its northern boundary, the polar front. The complexity of the Southern Ocean and its principle current, the Antarctic Circumpolar Current (ACC), create steep gradients in the physical and chemical properties of the water. Because it is a deep reaching current, the ACC transports nutrient rich waters that mingle, upwell, and then flow northward. These upwelled waters that form part of the polar front are rich in nutrients and CO2 which fuel phytoplankton growth in lower latitudes. Phytoplankton are at the base of the polar marine food web, and support a wide variety of Antarctic fauna like krill, fish, penguins, and whales. However, despite plentiful nutrients in the sea surface throughout the Southern Ocean, primary productivity is patchy at best.
The Southern Ocean is high nutrient-low chlorophyll region in which phytoplankton growth and biomass are limited by the micronutrient iron. Nutrients, upwelled by the ACC, are left unutilized by phytoplankton in the upper water column. These nutrient concentrations correspond to lower than expected values of chlorophyll concentrations, and consequently lower values of primary production. Iron is an essential element in the cells of phytoplankton, and is required for growth and maintaining life functions. Thus if more iron was available to phytoplankton, primary production would increase and blooms would be more consistent.
In addition to iron, light is an important factor in regulating productivity in the surface mixed layer. In the Southern Ocean, light availability varies daily and seasonally, and because the mixed layer depth can be very deep, phytoplankton can spend considerable periods of time below the sunlit euphotic zone. Although primary production in the Southern Ocean is highly variable in space and time, phytoplankton are responsible for the vast proportion of carbon fixation of CO2 into organic matter that is transported to deep ocean sediments. During iron fertilization experiments, where large amounts of iron are added to the surface waters, massive phytoplankton blooms occur.
Today, diatoms, a type of phytoplankton, dominate the Southern Ocean. Diatoms create their cell walls with silica which they extract from sea water in the form of silicic acid. Their cells are heavily silicified and can easily sink to the deep ocean, accumulating on the sea floor, and creating deposits of silica up to 1,400m thick. Iron limitation can cause diatoms to change their silicon to nitrogen uptake ratios. During periods of high iron input to the Southern Ocean, diatoms decrease this ratio which leaves excess silicic acid in the surface waters which circulate northward to the subtropics. During periods of low iron input diatoms extract more silica relative to nitrogen from the water to incorporate in their frustules, leaving surface waters depleted in silicic acid. In this way diatoms essentially control the silicon cycle and indirectly the global carbon cycle.
The most abundant and widespread species in the Southern Ocean is Fragilariopsis kerguelensis, which can reach up to 90% of the total diatom assemblage. F. kerguenlensis, a pennate diatom, is also highly responsive to iron enrichment experiments, in which they form the main component of phytoplankton blooms. When they die and sink to the deep ocean, F. kerguelensis exports silica and other organic matter to the sea floor. The underlying sediments and oozes are also composed mostly of F. kerguelensis. Because of its high abundances, wide distribution in the Southern Ocean, and its role in the silica cycle, F. kerguelensis is an ecologically important diatom species.
Despite the scarcity of iron and fluctuating light availability in the Southern Ocean, F. kerguenlensis are able to survive due to its ability to minimize iron requirements for cellular functions. Studies indicate that F. kerguelensis is a more competitive species, because it has the ability to reduce its cellular iron demands and is able to access a wide range of iron species in seawater. It is also believed that F. kerguelensis has the ability to store excess iron. In a phylogenetically similar species, Fragilariopsis cylindrus, a gene encoding the iron storage protein ferritin, has been identified.
In addition to minimizing iron requirements during times of iron stress, diatoms decrease their cell size and change frustule dimensions. Diatoms have cell walls, which are composed of two overlapping sections, called valves. Surrounding the valves are girdle bands that allow gas and nutrients to pass through to the cytoplasm. Decreases in diatom cell size, volume, and shape are a common response to a variety of environmental conditions. For example, in silicon and nitrogen-limited centric diatoms, valves become two times longer and half as wide, which might indicates the frustule is unable to divide properly. In previous studies, Pseudo-nitzschia spp. were shown to reduce their valve widths relative to their length under iron limitation. Likewise, other diatoms can decrease their cell volumes by as much as 50% when iron stressed. By increasing their surface area-to-volume ratio, they can maximize the number of nutrient transporter sites and increase nutrient uptake rates compared to their nutrient requirements. Studies show that F. kerguelensis decrease their cell dimensions when stressed, however, it is still unknown if valve morphology can be used as a proxy for iron limitation.
In the Marchetti Lab, our interest in F. kerguelensis will focus on identifying the molecular bases for physiological responses of different light levels and iron concentrations. One focus will be to determine if F. kerguelensis has iron responsive target genes, such as ferritin and ISIP2A, a gene that encodes for an uncharacterized iron starved induced protein. The goal will be to develop a molecular indicator, the F. kerguelensis Iron Limitation Index, which can be used to assess the iron status of F. kerguelensis in natural polar phytoplankton communities. Further work on F. kerguelensis will include valve morphology studies and comparative transcriptomics analyses. F. kerguelensis will be grown under a matrix of different iron concentrations and light levels with the goal to identify specific protein-encoding genes and metabolic pathways that are affected by iron limitation and to determine if valve measurements can be used as a proxy for iron limitation.




University Operator: (919) 962-2211 | © 2024 The University of North Carolina at Chapel Hill |